At Tamro, the distribution of medicine that requires refrigerated transport is closely planned and monitored. The packages are transported from the cold storage to pharmacies in specially made red boxes that are maintained at a level temperature with the help of smart technology.
Many products require refrigerated transport and an uninterrupted cold chain during the whole transport process from the factory to distribution companies, storage facilities, and finally, pharmacies. The cold chain must not be interrupted even under the most extreme conditions.
For medical products like this, the storage temperature must remain between two and eight degrees Celsius, even if the temperature outside the transport vehicle is plus or minus 35 Celsius, says expert pharmacist Miia Laukka from Tamro.
There are many types of medicine that require cold storage, such as insulin preparations, anti-cancer drugs or eye drops. Out of Tamro’s 9,000-product selection, 1,300 items require refrigerated transport.
Unloaded into temperature-controlled storage
When a truck carrying medicine that requires refrigerated transport arrives in Finland, usually by ship to Helsinki or Turku, it is like a refrigerator that moves on wheels. Transport boxes from different pharmaceutical companies may use different solutions, but all of them serve to keep the temperature constant. At this stage, the quality of the transport is still the responsibility of the pharmaceutical company that sent the products. Sometimes the whole container can be used as cold storage, sometimes just individual boxes.
When the truck arrives, it drives directly to our storage facilities and to a certain door. Behind this door is a cold unloading area, where even the unloading dock is equipped with cooling equipment. This way, heat cannot enter the unloading area even at the heat of summer, says Jani Kaikuranta, logistics manager at Tamro.
The medicines are unloaded in a specific, pre-defined order to ensure that any deviations in the transport process or packaging itself will be noticed right away.
The temperature data loggers, which are a type of memory stick that accompanies the cargo from start to finish, are an important part of quality assurance. They record any changes in temperature during the journey, Kaikuranta says.
The packages are inspected and their batch numbers are compared with the relevant order. The medicine will only end up on the storage shelves once everything has been double- and triple-checked.
A cold passage filled with advanced technology
From the incoming goods area, the medicines are transferred to a tall cold storage, which is called a cold aisle. The cold passage has 3,400 pallet slots. The medicine packages are transferred from pallets to these slots using an automatic conveyor system.
The cold aisle is filled with advanced technology, and there is a failsafe for every function: if one device fails, there is always an identical one ready to replace it immediately. A warehouse that is very critical to security of supply is expensive to build, but it is also a very stable environment. Instead of people, the products are moved from one place to another by computer-controlled automatic conveyors, Kaikuranta explains.
When the ERP (enterprise resource planning) system receives an order for medicines that require refrigerated storage, the next phase of the process begins, and the products begin to make their way through the refrigerated picking area all the way to the customer.
A robot replaces the cold accumulators
Even though the medicines are packed into transport boxes by human hands, robotics are also utilised in the picking process. The temperature-controlled transport boxes that are prepared especially for this purpose are an integral part of the refrigerated transport process. They are prepared for their journey by robots that replace the old cold accumulators in the boxes with new ones and measure their temperature. This reduces the risk of human error.
When a red transport box is ready, the collector places the products in the box. Once the products are in the box, a cold accumulator is placed on top of them, after which the lid is closed. Only after this does the box leave the refrigerated facilities, Jani Kaikuranta says.
The whole process takes place in a temperature of five degrees Celsius.
In the dispatch area, the transport boxes are sorted onto different pallets according to their destination. The pallets are then loaded on trucks that transport them all around Finland. The order of collection takes into account the distance and time it takes for the cargo to reach its recipient. The boxes that travel the greatest distance to the North of Finland are collected first.
A result of thorough development work
Tamro’s self-monitoring process is an important part of quality assurance. The cargo is always accompanied by loggers that measure the temperature in the box at regular intervals. Results are stored on a smart device for later inspection.
We have invested hundreds of working hours into the qualification testing of these boxes. The specially made refrigerated transport boxes have been exposed to both hot and cold temperatures in order to ensure their quality. In this way, we were able to develop a product that is among the best transport boxes in the field, says Tamro’s quality assurance pharmacist Elina Rintala.
According to tests, Tamro’s refrigerated transport boxes are able to maintain a constant cold temperature for up to 48 hours. In practice, the medicines are always transported to their destination within 24 hours, so that products that have been packed in the evening will have reached the ordering pharmacy by the following morning. The additional 24 hours make sure that even if something completely exceptional happens on the way to the pharmacy, the medicines will still remain at a constant temperature.
The innovation is a result of collaboration between Tamro and one of the company’s partners. Expert pharmacist Miia Laukka’s thesis project in 2014 was also an important stepping stone on this journey. The development work, which has gone on for several years, is a shared point of pride.
We are very happy that we have been able to do truly pioneering work here. On an European scale, our cold chain is first-class, says Elina Rintala.
At the request of the customers, the refrigerated transport boxes are always red. Other medicines are transported in green boxes. This ensures that the pharmacy will store the medicines appropriately and unload them immediately once they arrive.
CAPTIONS:
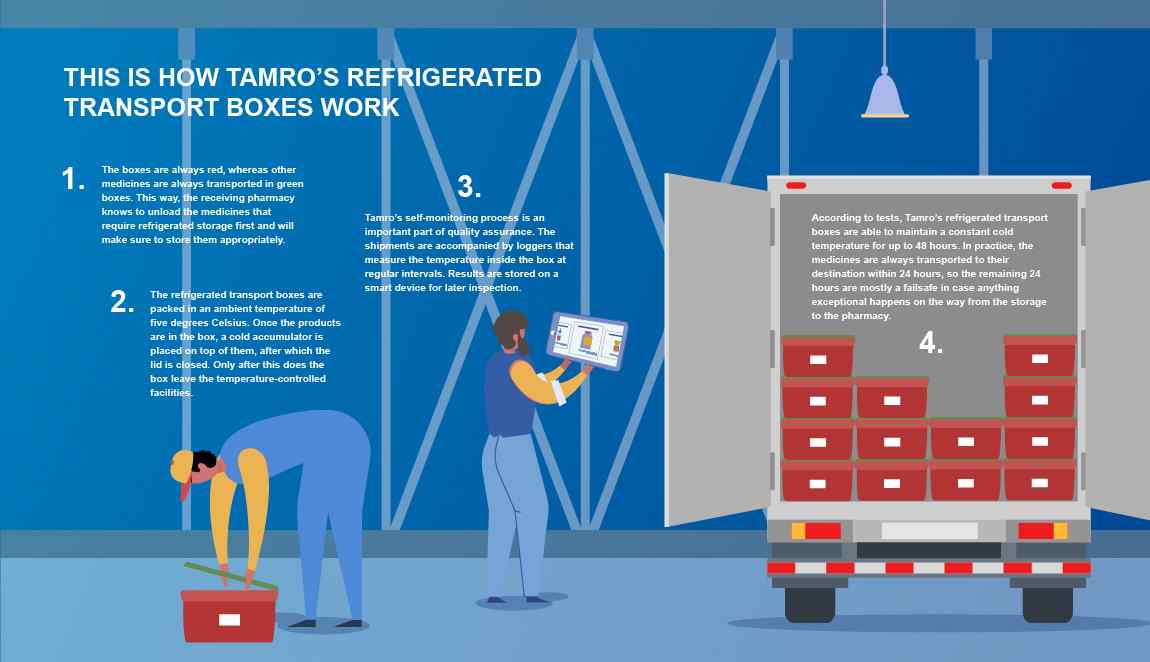
This is how Tamro’s refrigerated transport boxes work.
- The boxes are always red, whereas other medicines are always transported in green boxes. This way, the receiving pharmacy knows to unload the medicines that require refrigerated storage first and will make sure to store them appropriately.
- The refrigerated transport boxes are packed in an ambient temperature of five degrees Celsius. Once the products are in the box, a cold accumulator is placed on top of them, after which the lid is closed. Only after this does the box leave the temperature-controlled facilities.
- Tamro’s self-monitoring process is an important part of quality assurance. The shipments are accompanied by loggers that measure the temperature inside the box at regular intervals. Results are stored on a smart device for later inspection.
- According to tests, Tamro’s refrigerated transport boxes are able to maintain a constant cold temperature for up to 48 hours. In practice, the medicines are always transported to their destination within 24 hours, so the remaining 24 hours are mostly a failsafe in case anything exceptional happens on the way from the storage to the pharmacy.