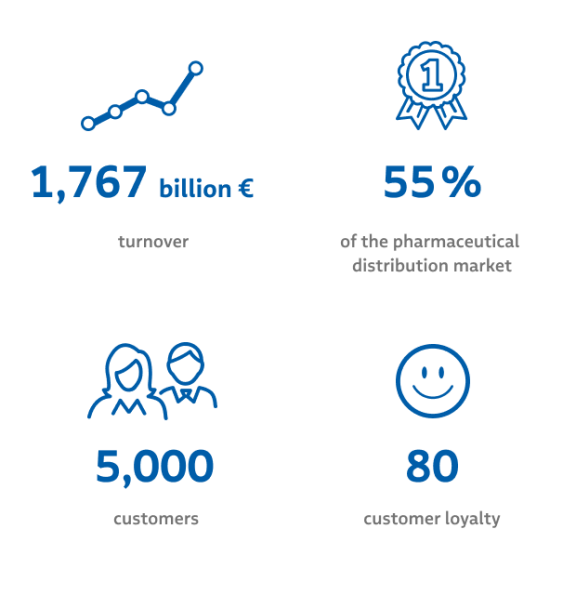
We efficiently and safely distribute pharmaceuticals and health products from the manufacturer to the consumer. We are the market leader in Finnish pharmaceutical distribution. The availability of products, accuracy and timeliness of deliveries and customer satisfaction are the crucial cornerstones of our operations and measured regularly for continuous development. Furthermore, we also transport medicines as emergency deliveries from the Tamro logistics centres to customers and patients – at any time of the day.
In Finland, pharmaceutical deliveries are handled as single channel distribution: A pharmaceutical wholesaler enters into a distribution agreement with a pharmaceutical company and is responsible for distributing all of the company's products to pharmacies and hospitals.
When it comes to the manufacturers of health products, we offer national single contract retail channels in addition to the pharmacy and hospital channels. We are a reliable partner to our retail customers with a reach of over 4,000 retail and health food shops in Finland. For example, the storage and distribution process for nicotine replacement products has been developed in cooperation with retail customers, and Tamro has been responsible for the distribution of these products in Finland since 2006.
Clinical IMP services
We are the largest importer and distributor of investigational medicinal products (IMP) in Finland. We have over 15 years of experience in clinical trials.
We import IMPs on behalf of the sponsor of the clinical trial and apply for import authorisations for special products from the authority.
The processing and storage facilities for IMPs meet the quality and regulatory requirements. We also handle products that require cool and cold storage, as well as narcotics, cytostatic agents and other IMPs that require special handling.
We deliver research medicines to all hospital pharmacies and research centres in Finland in accordance with GDP. Returns of IMPs are collected from research centres and stored pending a disposal permit or export. Upon receiving a written disposal permit, the IMPs are disposed of in accordance with local regulations.
IMPs stored at Tamro can be traced back to the individual package. The electronic system of clinical IMP services enables flexible reporting and also provides the customer with continuous visibility over the location of the IMPs. Tamro has a licence for the industrial manufacture of medicinal products issued by Fimea, which also allows the re-labelling and secondary packaging of IMPs under GMP. The batch release policy for orders is defined in a separate quality agreement.
Please contact our team of experts and we'll take action.

Kliiniset tutkimuslääkepalvelut
Expert service for special permit products
We offer pharmaceutical companies an expert service to deliver special permit products to those who need them.
In order to acquire one of these products, a special permit from the Finnish Medicines Agency Fimea is needed. Fimea can grant either a product-specific temporary permit or a patient-specific permit that the pharmacy requests on behalf of the customer.
Our service helps to ensure the continuity of the patient's medical care in cases where the availability of the authorised product varies.
- We can find anything from one package of a specific medication for a single patient to replacement products for high-volume pharmaceuticals.
- Many of the products are already in stock, whereas some are ordered when necessary.
- We look for alternative products within our extensive, international supplier network in Europe and the Northern America. We import the special permit product, contact the Pharmaceuticals Pricing Board to apply for reimbursement status and handle the customer communication, if necessary.
According to a 2021 survey conducted on regular and hospital pharmacies, customers are extremely satisfied with the service provided for the special permit products (overall grade 8.8 and NPS 67). Over 70 % of the respondents considered Tamro to be their primary contact in a situation where they need to order medications that require a special permit.
Lauri Itkonen, Pharmacist at the Porvoon Uusi Apteekki pharmacy: "Tamro’s special permit product team are like our right hand in these matters. Tamro has a lot of information about the products that we at the pharmacy do not have or are not even supposed to have. Contacting the customer care team is easy, since they have such an active and solution-oriented attitude. Communicating via email is convenient, and the team usually responds quickly."
Sami Huilla, in charge of Pfizer’s hospital operations in the Nordic countries: "Although we at Pfizer have invested heavily in production plants and production chain, sometimes we are faced with a global shortage of a certain raw material. During such times, we rely heavily on Tamro’s expertise and networks."
Please contact our team of experts and we'll take action.



Robert Oksanen

Petteri Lajolinna

Tomi Hakala





